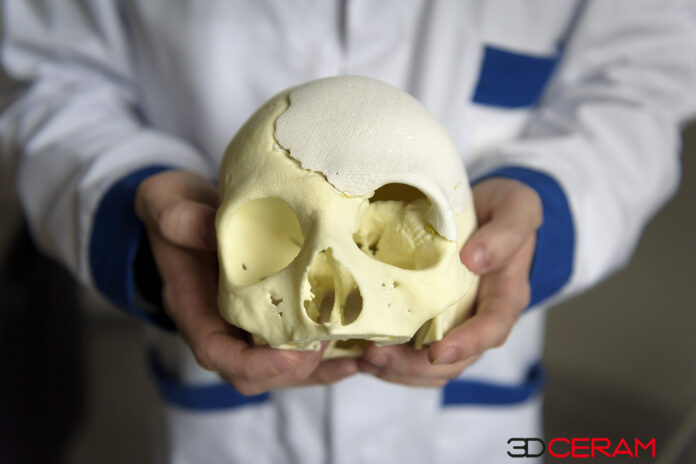
As explained before, one vertical industry where ceramic 3D printing is making great strides is the healthcare industry. Surprisingly, while implants constitute one of the largest use cases for technical ceramics, all of them are not given the same attention. 3D printed cranial implants for instance, are living the shadow of dental or orthopedic counterparts. While no exact figures justify this argument, one tends to notice that their manufacturing process is often highlighted with the use of Titanium or PEEK 3D printing. 3DCeram, a machine manufacturer and ceramic 3D printing service provider that has been continuously exploring new applications with its SLA 3D printing technology, is bringing a new approach to this table. Acknowledged for its ability to support the production of small series of bone substitutes (intervertebral cagesand tibial osteotomy wedges), 3DCeram’s technology can also be used to manufacture cranial implants. Dr Joël Brie, Head of the Maxillo-facial Surgery Department at Limoges University Hospital has been working with the ceramic 3D printing company on craniofacial skeletal applications, with specific shapes. In the article below, Dr. Joël Brie and Christophe Chaput, 3DCeram CEO, sat together and discussed how Brie has been able to overcome their main challenge – the perfect reconstruction of complex shapes in cranial implants – using 3DCeram’s AM technology. The conversation also shed light on the journey that leads Brie and his team to use 3D printing, and the specific pathologies at the heart of this journey.
Christophe Chaput (CC): When did you start using 3D printing ceramics and what are the different phases that led you to this technology?
Joel Brie (JB): We started in 2005. To bring an implantable medical device to market, it’s necessary to go through several stages of clinical trials. One must first check the feasibility. This means one must validate the process and the possibility of using it to corroborate the fact that it can deliver good results. This includes the effectiveness study for which we have to increase the number of patients to reach an absolute proof of efficacy. Once this feasibility study has been checked, there is a CE marking requirement one must meet.
While we were obtaining the CE marking, we conducted up to 24 case studies. The insights showed that we were able to produce evidence of effectiveness and safety. Out of the 24 cases, no cranial implant has so far been removed due to infection or complications.
CC: What indications led you to the manufacture of a tailor-made prosthesis/implant?
 JB: For me, large losses of bone substance that cannot be filled with the patient’s own bone constitute the main indication. For the skull, it is essentially the cranium-plastic; in other words, a loss of bone substance of the cranial arch which will be greater than 100 square centimeters. We can say that up to 100 square centimeters, we can manage with other processes that are real acceptable choices like autologous cranioplasty* or titanium grids with hydroxyapatite cement. Beyond 100 square centimeters, the custom prosthesis is absolutely essential. That being said, from 25 to 100 square centimeters, one can either use the custom prosthesis or other systems. But the custom-made prosthesis is the best choice as it delivers better results.
JB: For me, large losses of bone substance that cannot be filled with the patient’s own bone constitute the main indication. For the skull, it is essentially the cranium-plastic; in other words, a loss of bone substance of the cranial arch which will be greater than 100 square centimeters. We can say that up to 100 square centimeters, we can manage with other processes that are real acceptable choices like autologous cranioplasty* or titanium grids with hydroxyapatite cement. Beyond 100 square centimeters, the custom prosthesis is absolutely essential. That being said, from 25 to 100 square centimeters, one can either use the custom prosthesis or other systems. But the custom-made prosthesis is the best choice as it delivers better results.
[*Autologous cranioplasty means that the bone that is removed at the time of craniectomy has been preserved for future implantation.]
CC: What are the pathologies leading to this type of surgery?
JB: I would say, the flaps of the compressive that are not going back into place. It is a head trauma with an increase in intracranial pressure which causes a cerebral edema that develops after the trauma. To save the patient, it is necessary to remove the skull arch or remove up to the hemi-skull. The decompressive flaps can be very large; they are saved at the bank of tissues to be implanted when the edema will have disappeared.
CC: How long can this take?
JB: Maybe several months. Once the brain is back in its place, we will try to put the flags back. The problem is that at the bank of tissues, the flap could eventually be contaminated during the manipulation to store it. Samples collected before replacing the skull vault could reveal that the liquid in which it was stored is contaminated, in which it case, it cannot be used.
CC: What are the advantages of ceramics over other materials that are available for skull implants today?
 JB: There are three main families of biomaterials today: first generation, second generation and third generation.
JB: There are three main families of biomaterials today: first generation, second generation and third generation.
Put simply, the first generation of biomaterials is a range of bio-inert materials. This means, that the organism accepts them, but with no interaction between the material and the organism, there is no osteointegration. The second generation of materials will interact with the living environment. For example, the osteo-induction is a second generation implant property. That means that we can induce bone manufacturing by interaction with the body. This is the characteristic of calcium phosphate and all materials made up of calcium and phosphate, like hydroxyapatite, phosphate tricalcium, biphasic, etc.
So, if we create porous parts, there will be a colonization of the implant by bone tissue. With polymer materials, there is no colonization of the implant. The implant is massive, it is placed and it is firmly fixed to the bone, but there is no bio-integration of the implant as such. In such case, there is a bigger risk of a rejection, infections that are much more important because the implant is rigidly fixed with some plates that are a little thick, and it will never be considered as an element of the body. It will never be truly bio-integrated.
The first cranioplasties were done with polymethyl methacrylate cement. This is the cement that is used to seal hip prostheses in orthopedics with a failure rate reaching 20%, because of infectious problems.
CC: On 3D printed implants produced by 3DCeram’s technology, have you had any cases of rejection?
JB: None. The implant that we were using has a rather particular structure. Since we know very well that the colonization of an implant is limited, it does not go all the way to the bottom. If the implant is very massive, the colonization by bone tissue will take place over the first centimeter, but not beyond. So, with this in mind, we decided to have a porous part at the periphery, to make the link with the living bone tissue. This is what you can see between the fixation holes. This prosthesis is perfect as there is a dense part in the middle, with a surface porosity that is important enough for the soft tissues to be able to attach to the prosthesis. These dense parts that lie in the periphery were the place of the fixation holes of the prosthesis and between the fixation holes there is a porous area. In fact, our study revealed that, after one year, 75% of the periphery was bounded. You could clearly see the fusion between the surrounding bone tissue and the prosthesis.
There is a primary fixation that was done with absorbable sutures and the secondary fixation of the implant was the result of this osteointegration. These fixations enabled the creation of bone bridges that facilitated the integration of the implant and its steadiness.
With a polymer implant on the other hand, you do not have a bone/implant bond, you have a membrane that forms between the two. The stability is just due to the system of fixation, the plates and screws that hold it in place, so there can be micro movements. Those micro movements at the interface between the implants and the native bone may cause rejections.
Reconstructing the skull is not only a cosmetic or protective function. It also improves the functioning of the brain. The right 3D printed implant might therefore help in that process.
CC: What would the results look like with titanium implants?
Well, we wouldn’t be able to get the thin parts we got with ceramics on the external part of the implant with titanium. The titanium plates (manufactured with a conventional manufacturing process) deliver 2mm of thickness, so the patient can feel the screws through the skin of the forehead – which is not ideal. Not to mention that, some titanium ions can be released on the periphery, causing an inflammatory reaction around the implant.
With ceramic skull implants however, we were putting resorbable sutures, that went through a hole so there was no perception of any material. That is why the result was close to perfection. The thin parts facilitate osteointegration and the fixation material was not perceptible at all. It’s the ideal continuum between the implant and the bone.
The content of this conversation has been edited to meet 3D Adept Media’s editorial requirements.