The article below has been updated to provide further info about Evonik VESTAKEEP® i4 3DF PEEK material
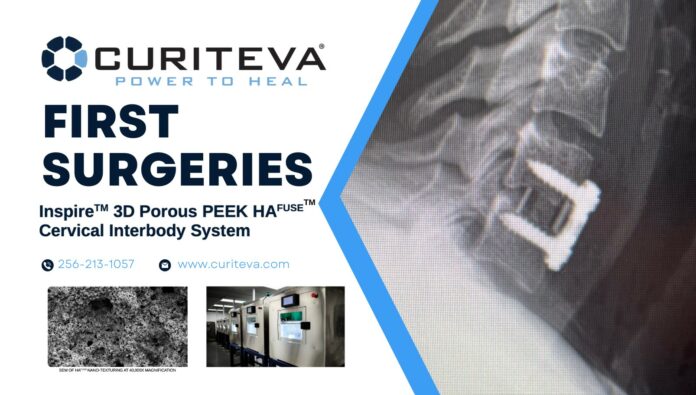
Two months ago, medical technology company Curiveta was granted FDA clearance for a 3D printed PEEK implant, the Inspire Porous PEEK Cervical Interbody System with HAFUSE Technology. Developed in-house using a patented FFF 3D printer designed, programmed, and built by Curiteva, the Inspire platform is manufactured utilizing the Evonik VESTAKEEP® i4 3DF PEEK high-performance polymer.
“Structure drives biology and the lattice PEEK architecture enabled by Curiteva’s 3D printing process represents an exciting advancement in spine, orthopedics, and neurosurgical procedures which involve any type of biologic implant“, Alex Vaccaro, MD, PhD, president of Philadelphia-based Rothman Orthopedic Institute shared.
Since the FDA clearance, surgeons have been able to complete first surgeries with the new product.
As Kevin Foley, professor of neurosurgery, orthopedic surgery and biomedical engineering at the University of Tennessee Health Science Center, implies, the Inspire porous PEEK technology checks all of the boxes for an ideal interbody implant: fully interconnected porosity, modulus of elasticity equivalent to cancellous bone, strong biomechanical properties, radiolucency, and a bioactive surface for osseointegration.
Added to these boxes a pore size distribution, and nano-surface architecture, and the implant enhanced with HAFUSE nano-surface topography enables the most effective synthetic allografts. It also creates an optimal environment for osteoprogenitor cells to move throughout the implant enhancing bone healing (fusion) and reducing risk of subsidence.
What is this PEEK filament biomaterial designed for medical 3D printing?
Designed especially for use in additive manufacturing processes, Evonik’s VESTAKEEP® i4 3DF comes in filament form and meets stringent requirements of ASTM F2026, which is the standard for PEEK polymers approved for use in surgical implant applications.
“Innovative developments like our VESTAKEEP® i4 3DF PEEK filaments, are designed for the utmost biocompatibility, biostability and x-ray transparency – making them excellent materials for orthopedic and maxillofacial surgery,” says Marc Knebel, head of Evonik’s Medical Devices & Systems market segment.
Evonik’s products (high-performance polymers and additives) have been used in 3D printing applications for more than 20 years. In addition to these implant grade filaments, the company produces a testing-grade PEEK filament that offers the same properties without the documentation needed for surgical implants. Its other 3D printing materials are used in highly demanding environments, and include resins suitable for photocuring and powders ideal for sintering -based manufacturing processes.
Curiveta plans a full commercial launch in the United States later this year.
Remember, you can post free of charge job opportunities in the AM Industry on 3D ADEPT Media or look for a job via our job board. Make sure to follow us on our social networks and subscribe to our weekly newsletter : Facebook, Twitter, LinkedIn & Instagram ! If you want to be featured in the next issue of our digital magazine or if you hear a story that needs to be heard, make sure to send it to contact@3dadept.com.