Brain tumours are certainly the most devastating type of disease. Parkinson is the result from the break-down of dopamine producing neurons in the brain. So far, no treatment has ever enabled to completely heal a patient. Among the researches that have been undertaken in this regard, a joint Phase 1-2 clinical study carried out with Herantis Pharma plc, investigates cerebral dopamine neurotrophic factor (CDNF) as a treatment for this disease.
According to experts, the study’s repeated delivery regime would enable a prolonged therapeutic window and thereafter achieve the potential neuroprotective and neurorestorative actions of CDNF.
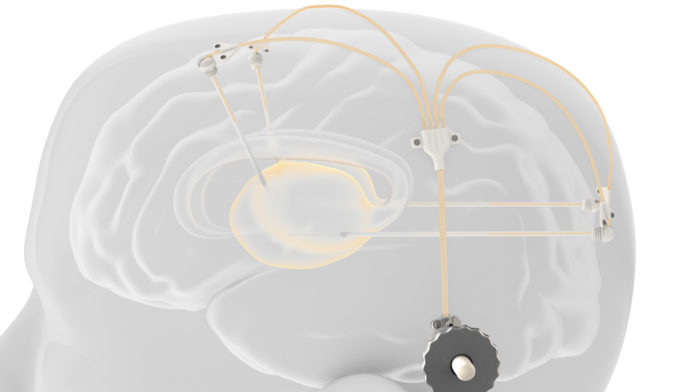
To make it possible, the research team leverages Renishaw’s drug delivery system. The device comprises of up to four catheters, that can be implanted into target areas within the brain.
“The catheters are accessed via a 3D printed titanium transcutaneous port implanted behind the patient’s ear. Drug-filled infusion lines are connected using an MRI compatible application set, which repeatably locates onto the port. Retractable needles extend through a septum in the port to enable therapeutics in the external infusion lines to be infused through the implanted catheters” explains Renishaw.
Normally, a patient would have received the reimplantation of new catheters for each infusion. With the novelty of Renishaw design, it is now easier for patients to receive infusions in an out-patient setting.
Rupert Jones, Managing Director of Renishaw Medical, said, “The results of this trial and the performance of Renishaw’s drug delivery system are promising for the many people with Parkinson’s disease and I would like to take this opportunity to thank the trial participants for making this possible.”
Indeed, first results show that it is possible to accurately predict the placement of the drug delivery system and its positive performance and safety. As patients receive ongoing monthly infusions of CDNF utilizing this device, both teams will continue to analyze the data of this study.
“These results allow us to build towards CE marking of Renishaw’s device so that further neurodegenerative and neuro-oncological conditions can benefit from our technology. We see our device as an enabling technology that facilitates the reliable and repeated delivery of therapeutic agents direct to targets deep within the parenchyma, as part of a paradigm shift in the way treatments of neurological disorders and brain tumours are progressing”, conclude Rupert Jones.
Remember, you can post free of charge job opportunities in the AM Industry on 3D ADEPT Media or look for a job via our job board. Make sure to follow us on our social networks and subscribe to our weekly newsletter : Facebook, Twitter, LinkedIn & Instagram ! If you want to be featured in the next issue of our digital magazine or if you hear a story that needs to be heard, make sure to send it to contact@3dadept.com