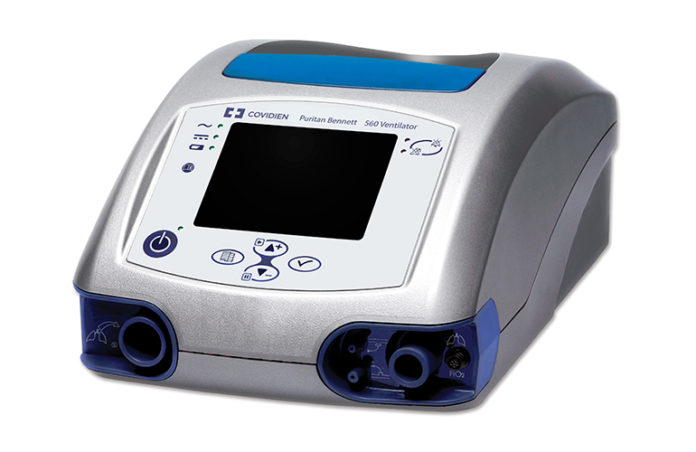
Medtronic plc has shared design specifications for the Puritan Bennett™ 560 (PB 560) to enable participants across industries to evaluate options for rapid ventilator manufacturing.
This decision is consistent with the recent FDA Guidance and in accordance with the public health and medical response of governmental agencies globally.
According to the global medical technology company, many parts of this ventilator could be produced using additive manufacturing. The PB 560 ventilator is a compact, lightweight, and portable ventilator that provides airway support for both adults and children. It can be used in clinical settings and at home and provides mobile respiratory support.
It’s already been a decade that the device is commercialized and sold in various countries around the world. According to the manufacturer, the product technology and design makes it an ideal candidate for a diverse range of care settings.
“Medtronic recognizes the acute need for ventilators as life-saving devices in the management of COVID-19 infections. We know this global crisis needs a global response. Over the past few weeks, we have ramped up production of our Puritan Bennett™ 980 ventilators. But we also know we can do more, and we are,” said Bob White, executive vice president and president of the Minimally Invasive Therapies Group at Medtronic. “By openly sharing the PB 560 design information, we hope to increase global production of ventilator solutions for the fight against COVID-19.”
So far, Spain has approved a medical 3D printable ventilator, that could respond to the demand of the healthcare industry but there is no information about, whether or not, the CAD files will be shared globally. Italian start-up Isinnova, has on the other hand, turned a snorkeling mask into an emergency respiratory mask, but due to the fact that this solution has not been certified by medical authorities, its “usage by the patient is subjected to the acceptance of use of an uncertified biomedical device, by providing a signed declaration.”
The critical role of ventilators in the management of patients with severe respiratory illness, such as COVID-19, is no longer a secret. And for a medically-approved ventilator, this contribution from Medtronic amid this pandemic is the first and most impressive one of its kind.
PB 560 product and service manuals, design requirement documents, manufacturing documents, and schematics are now available at Medtronic.com/openventilator. The PB 560 design specifications are available today, software code and other information will follow shortly.
Remember, you can post free of charge job opportunities in the AM Industry on 3D ADEPT Media or look for a job via our job board. Make sure to follow us on our social networks and subscribe to our weekly newsletter : Facebook, Twitter, LinkedIn & Instagram ! If you want to be featured in the next issue of our digital magazine or if you hear a story that needs to be heard, make sure to send it to contact@3dadept.com COVID-