
“…any improvement that we can bring on that formulation can improve their quality of life”
A rare or orphan disease is any disease that affects a very small percentage of the population. In Europe is defined as rare when it affects fewer than 1 in 2000 people. In the US is defined as fewer than 200,000 American at any given time. It is so rare that there is a lack of a market large enough to gain support and resources for discovering treatments for it.
However, this issue of rare diseases arouses the interest of some pharmaceutical companies such as Cycle Pharmaceuticals which has made it its core business. Founded 6 years ago by James Harrison (now Executive Chairman of the Board of Directors), it is run by Antonio Benedetti, CEO.
Accompanied by Steve Fuller, Business Development Director of Cycle, Antonio Benedetti talks about the difficulties faced by patients who suffer from rare diseases today, but above all, we talked about one of the solutions that the company proposes to reduce these obstacles and improve the quality of life of patients.
This solution which results from a partnership with Aprecia Pharmaceuticals, a company specialized in the 3D Printing (3DP) of drugs, explores the possibilities offered to patients with rare diseases. Possibilities that can drastically improve their quality of life and raise the interest of the healthcare industry.
Tell us more about Cycle Pharmaceuticals
Cycle is mainly focused on three business areas:
– Improving drugs – optimising an existing drug. A patient can take a treatment for a limited amount of time, dealing many times with suboptimal formulations (due to big capsules or constant injections).
This can be handled if it is for a short amount of time, but if the patients have to deal with a life-long treatment and has to take the medication many times per day for all their lives (from childhood to adulthood), then any improvement that we can bring on that formulation can improve their quality of life. We look at improving formulations to address patient’s unmet needs.
– Repurposing drugs – creating a new indication for an already existing drug. We are working very close to academics and Ivy League universities with which we are focusing on various scientific and technological projects.
– Generics – reinstating generic drugs, previously available in the market, improving market accessibility
These three areas of focus are underpinned by formulation technology – creating new drug delivery technologies to improve the efficacy and effectiveness of drugs, allowing Cycle to give patients a greater freedom and choice.
What about the partnership with Aprecia?
The partnership with Aprecia aims at bringing a novel technology, such as 3DP, in our Improving Drugs business area, focusing in rare diseases.
Aprecia has developed a first in class 3DP technology called ZipDose®.
This technology is the first approved by the FDA and offers two valuable attributes for treating rare diseases: a tablet that is fast melting (desintegrating in seconds) and the ability to include a large amount of drug in a single tablet, with Aprecia being the only company that can include such high doses of API in one tablet using this novel technology.
In other words, you can have more than one thousand milligrams in one single tablet that you can put on your tongue and with a little bit of water, it will melt in a matter of seconds.
We can then address two main issues:
– The first one is to reduce the number of tablets per day (pill burden). So, , for example, instead of taking 10 tablets of 300mg each of a specific medicine, they could be taking only 3 fast melting and easy to swallow tablets.
– For all the patients who suffer from dysphagia (difficulty to swallow), it becomes much easier to take a drug which is fast-melting in the mouth.
We have already triggered the first development with Aprecia and we look forward to be on our patient’s hands in 2 or 3 years.
We are going to develop other products with Aprecia, so this product is the first of a long and promising pipeline.


What are the main challenges you may encounter in this collaboration?
We are partnering with a top class company, so we expect more excitement than challenges.
Where do you commercialize your drugs?
We are a global company and we are already commercializing and distributing our products around the world. However, with regard to Aprecia’s partnership, we will first start in the US and Europe. These continents are our main objective.
What do you think about the use of the 3D printing technology in the manufacturing process of these orphan drugs?
The 3DP technology being used here allows us to produce an oral dispersible tablet that disperses much faster than other technologies, and can have much more drug per tablet. These properties are very important in the orphan drug world where patients often have to take lots of tablets every day, and often from a young age where swallowing is a problem.
The Aprecia 3DP manufacturing process is the only one approved by the FDA, and has shown none of the control problems that are a concern in other 3DP methods.
What are your prospects of development?
Cycle is a fast-growing company. We have already launched our first two drugs:
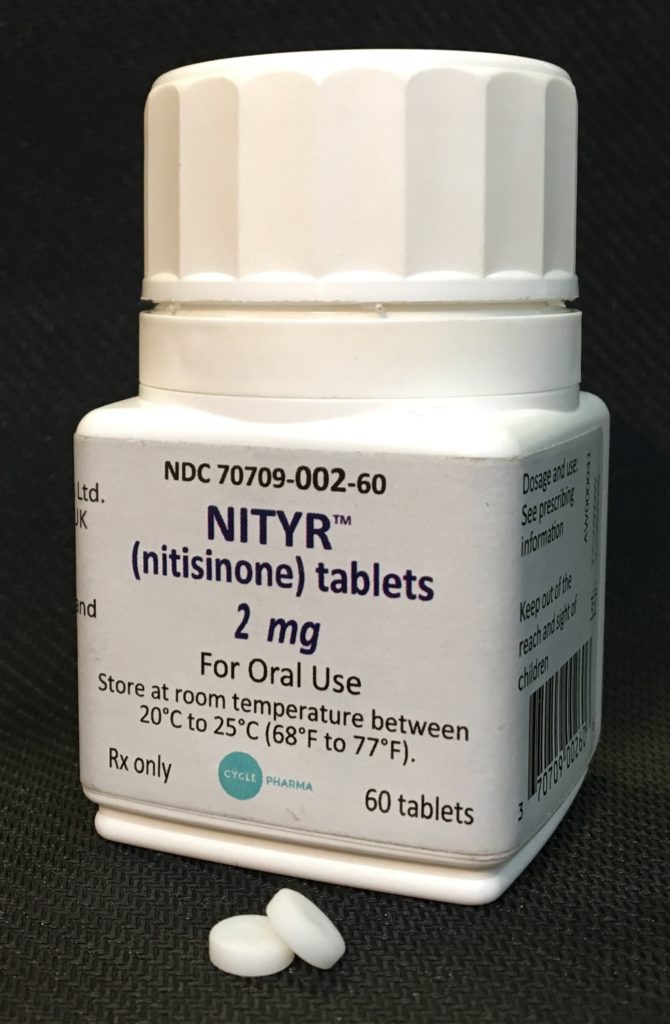
Nityr™ (nitisinone tablets), a new treatment option with an improved formulation for patients with tyrosinemia type 1, an ultra-rare condition;
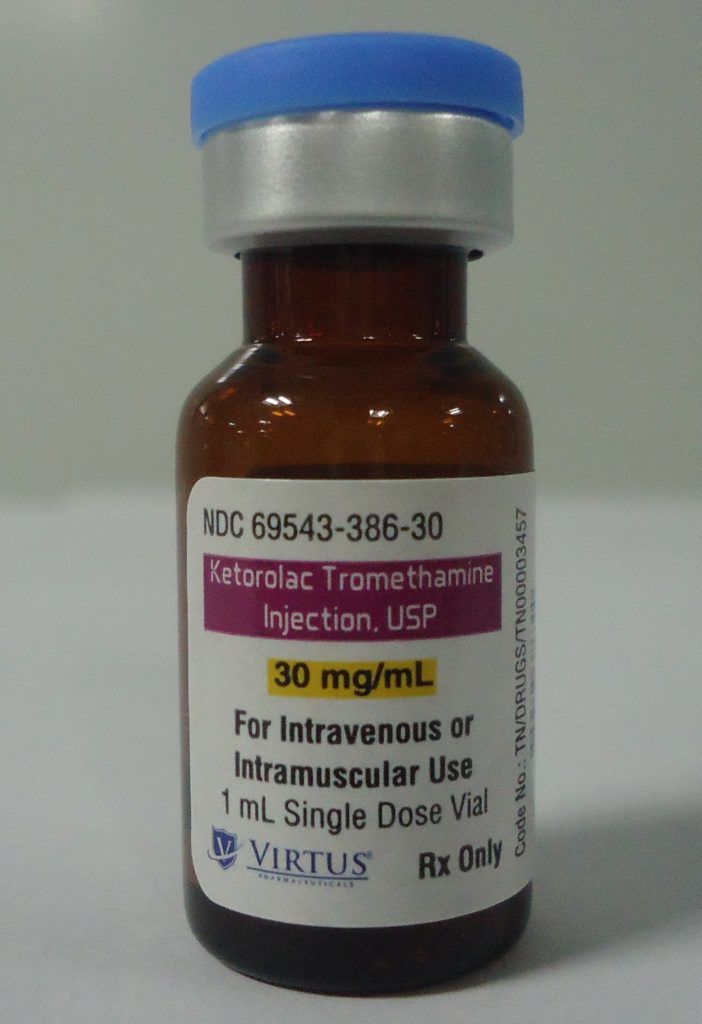
Ketorolac Tromethamine Injection, a non-steroidal anti-inflammatory drug and the first in our generic pipeline, brought to the market to address shortages currently preventing patients from receiving essential treatment.
We have a strong pipeline with new launches expected in 2018.
Do you have any last words?
We have a lot of work to do, the most exciting part is yet to come. We are confident about partnering with a global leader such Aprecia Pharmaceuticals.
For further information about 3D Printing, follow us on our social networks and subscribe to our newsletter!
//pagead2.googlesyndication.com/pagead/js/adsbygoogle.js
(adsbygoogle = window.adsbygoogle || []).push({});






