Materialise was granted FDA clearance for its software dedicated to 3D printed anatomical models for diagnostic use. It’s been three decades that Materialise is working on medical solutions to improve patient care, therefore the company has already a certain experience in medical 3D printing. In collaboration with Siemens, the Belgian company has brought its Mimics inPrint software to hospitals around the world. The software enables preparation prior to operation and the manufacturing of models for diagnostic purposes.
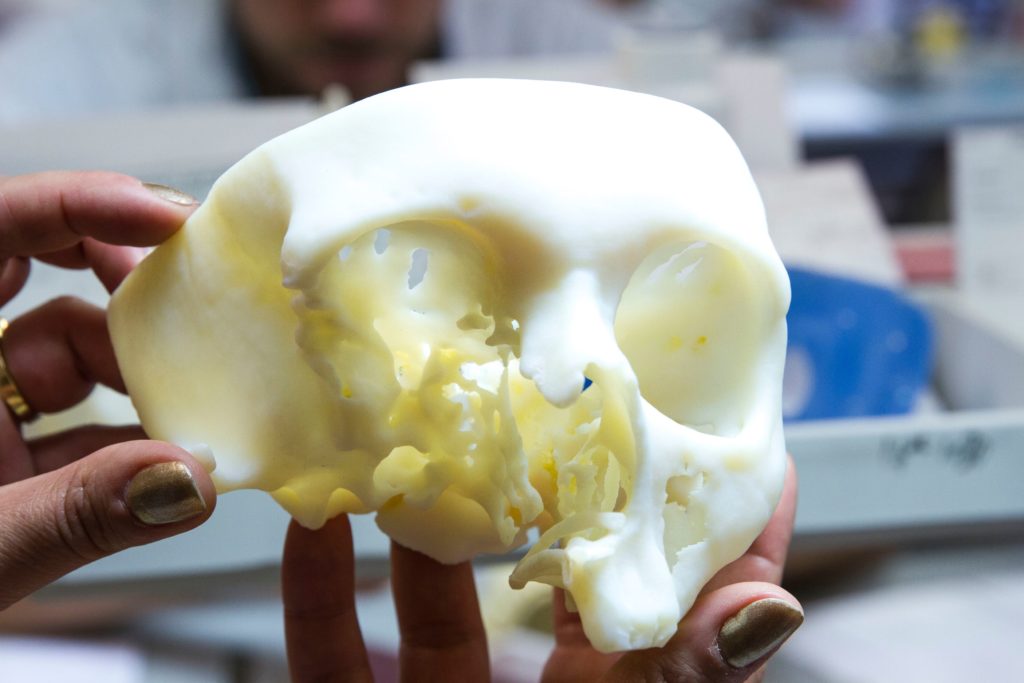
Furthermore, this month, the company unveiled the TRUMATCH® Personalized Solutions Shoulder System. The system is simply a 3D printed guide for shoulder surgery that enables surgeons to be better prepare before entering the theatre room.
Hospitals, therefore are increasingly aware of the added value 3D printing can bring to personalized patient care. One notes that a certain number of hospitals have already integrated medical 3D printing in their services.
FDA clearance
In August 2017, the FDA announced that software intended to create output files used for printing 3D patient-specific anatomical models which are used for diagnostic purposes, is a class II medical device and requires regulatory clearance.
The FDA clearance supports the creation of point-of-care 3D printing facilities in hospitals. In addition to pre-op planning, anatomical models also aim to enhance education and communication between multidisciplinary teams and with the patient.
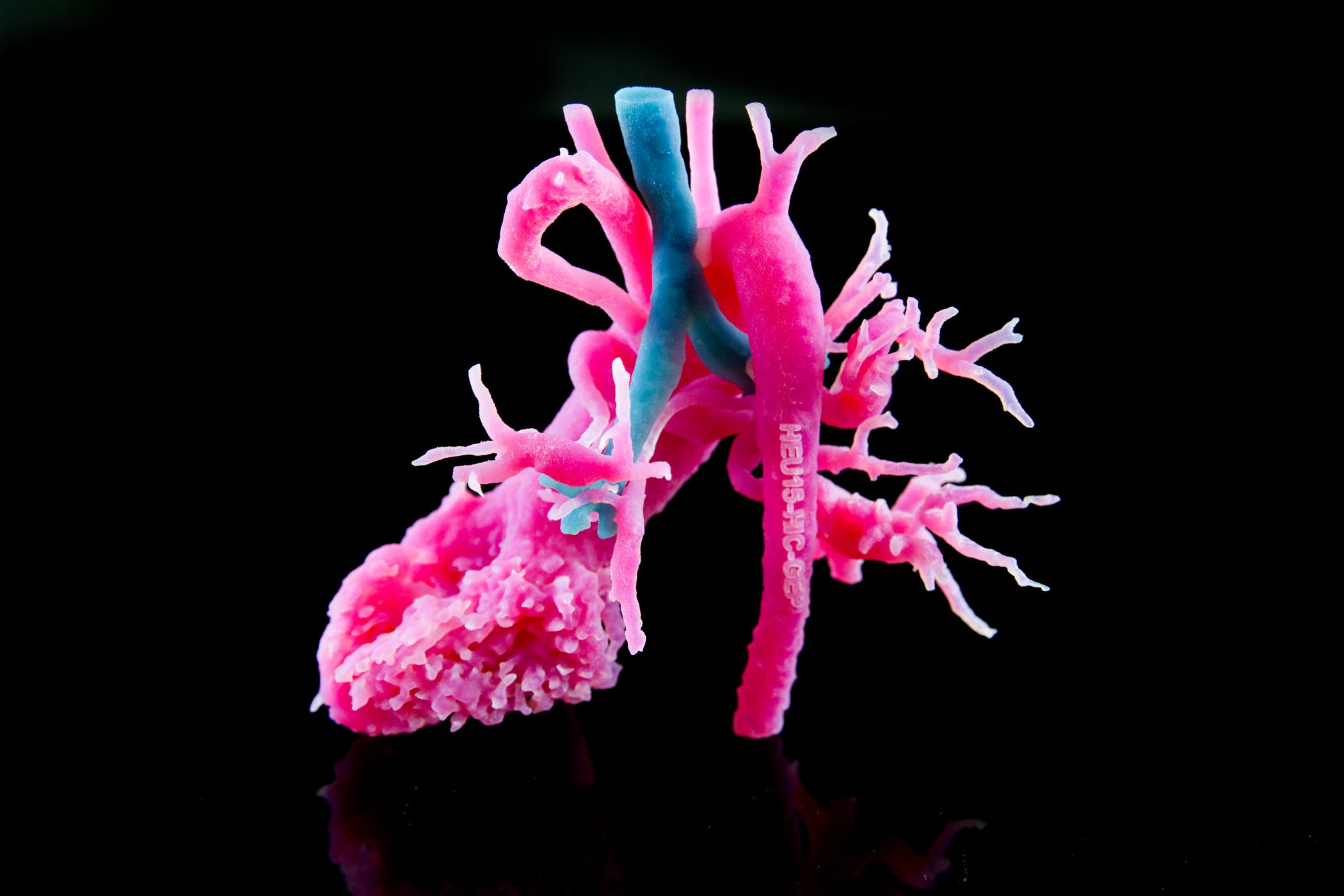
Wilfried Vancraen, Materialise CEO, said about the FDA clearance: “The FDA clearance for our Mimics inPrint software will support the adoption of 3D planning and printing in U.S. hospitals and the creation of point-of-care 3D printing facilities.”
For further information, follow us on our social media and subscribe to our newsletter!
Would you like to be featured in the next issue of our digital magazine? Send us an email at contact@3dadept.com
//pagead2.googlesyndication.com/pagead/js/adsbygoogle.js
(adsbygoogle = window.adsbygoogle || []).push({});