While within the 3D printing industry, 3D printed implants are strongly recommended, the Belgian Federal Knowledge Center for Healthcare (KCE) reported that it is difficult to draw long-term safety of 3D applications for the patient.
These risks and challenges faced by the medical industry have been evaluated for a specific range of countries: Luxembourg, UK, France, Belgium and more. The results featured in this article concern Belgium. Therefore, we did not verify the validity of these assessments for other countries. The report was made while taking into account the point of view of a wide range of 3D printing companies, hospitals and researchers. However, even though they did not always agree on the different focus of the report, KCE is the only one responsible for these results.
KCE laid emphasis on high-risk medical devices because they present a bigger potential risk for the patient and are often the most expensive. However, as far as process of 3D printed devices are concerned, it should be noted that the number of steps for each device depends on several factors, such as the complexity of the device or whether (parts of) the 3D printing process is outsourced.
Effectiveness and safety of 3D printing technology for medical indications
The case of custom-made implants
Experts randomized 13 patients with distal intercondylar humeral fractures to undergo surgery using either conventional plates (N=7) or 3Dprinted plates.
The 3D-printing group had a significantly shorter mean operative time (70.6 +/- 12.1 min) than the conventional plates group (92.3 +/- 17.4 min; p=0.026)). After a mean follow-up of 10.6 months, there was no significant difference between groups in the rate of patients with good or excellent elbow function, although the 3D-printing group saw a slightly higher rate of good or excellent evaluations (83.1%) compared to the conventional group (71.4%). One patient in the conventional group experienced intraoperative traction injury of the ulnar nerve. The injury resolved after 3 months of rehabilitation. No wound infections or other complications were observed.
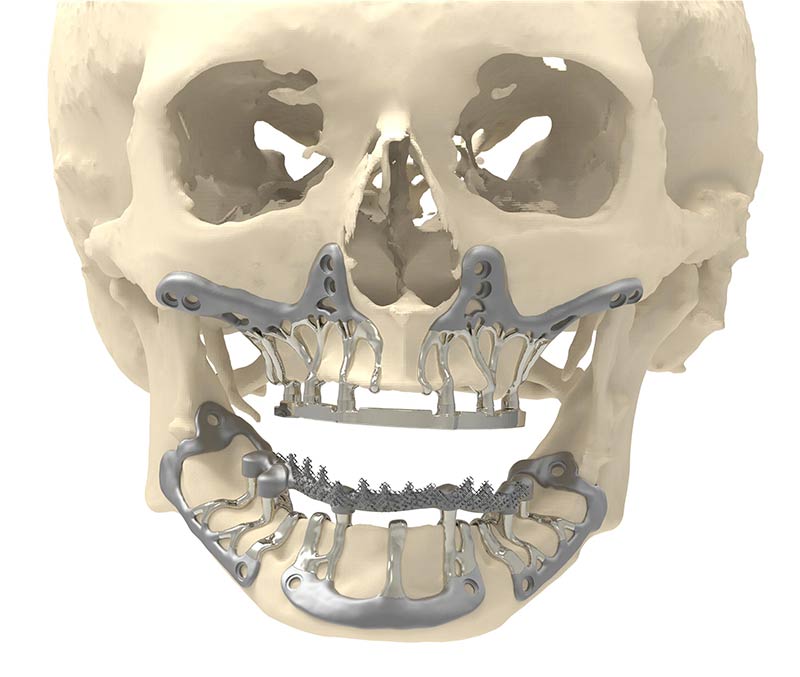
Other applications
For other indications such as surgical guides for orthopaedic surgery other than total knee arthroplasty, surgical guides for maxillofacial surgery, models for surgery planning or implant shaping, the trials that have been carried out were small and/or did not show significant effects.
In the end, researchers were not able to draw conclusions about the effect of 3D applications on (long-term) patientrelevant outcomes (such as rehospitalisation and quality of life). Last, it is hard to determine or talk about a long-term safety of 3D applications for patients.
3D printing technologies are advancing and increasingly become accessible, certification processes are increasingly being standardized for 3D printed parts, but a little doubt remains regarding the stated benefits of 3D printed implants.
You can read the whole report here.
For further information about 3D Printing, follow us on our social networks and subscribe to our newsletter!
//pagead2.googlesyndication.com/pagead/js/adsbygoogle.js
(adsbygoogle = window.adsbygoogle || []).push({});