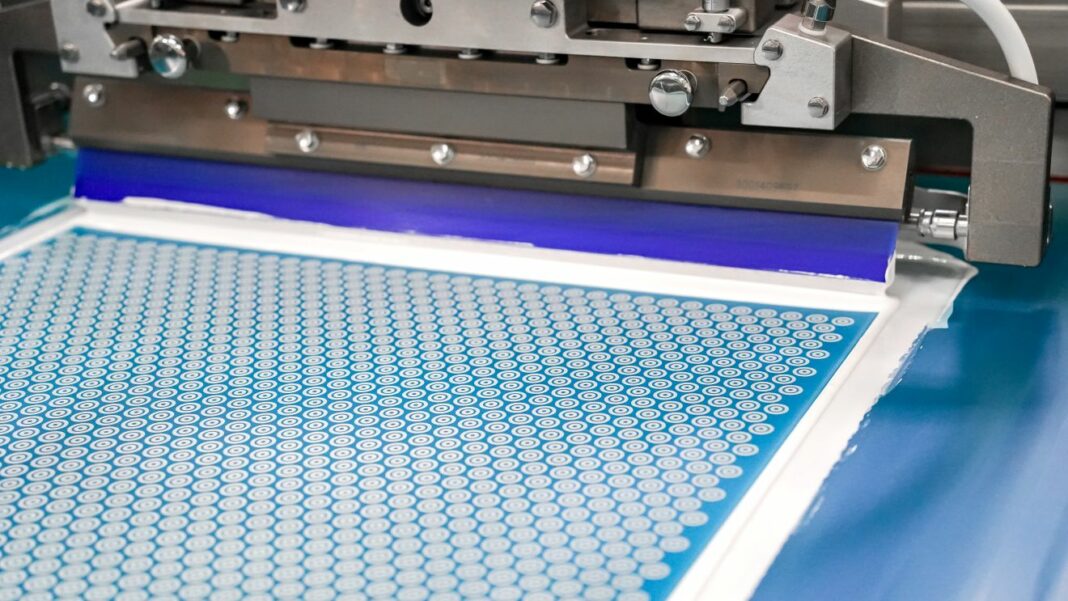
In a recent discussion on technical ceramics, high-performance variants emerged as promising candidates for 3D screen printing. Given its niche appeal, this manufacturing process is still the domain of a few specialized companies. We recently caught up with Exentis Group AG to explore its potential in healthcare and pharmaceutical applications—and to understand just how close we are to commercializing viable solutions.
One of the first companies that drew our attention to 3D screen printing and its potential for pharmaceutical applications was Laxxon—back in 2022. Fast forward to 2025: the ecosystem of specialists using 3D screen printing has expanded to include organizations like Fraunhofer IFAM Dresden and Axenoll Life Sciences Ltd. The truth is, 3D screen printing is still a relatively new manufacturing approach and hasn’t yet gained the long-standing reputation that other additive manufacturing processes enjoy. Currently, there is no universally established standard definition for “3D screen printing.” The term is used in various contexts, leading to differing interpretations across industries. In additive manufacturing and industrial applications, “3D screen printing” describes a process that extends traditional two-dimensional screen printing into the third dimension. This involves depositing successive layers of material through a screen to build up a three-dimensional object.
To understand the capabilities of this technology as a whole, it’s important to identify what makes the uniqueness of each process.
Designed specifically for large-scale pharmaceutical production, Exentis’ 3D screen printing technology supports mass production at an industrial scale. The process is said to produce up to 200 million pharmaceutical tablets per year, depending on tablet size and formulation complexity, and up to 50 million medical parts per year, including implants and ultra-fine technical ceramic components. Its key applications remain pharmaceutical tablets (customizable release profiles and multi-drug formulations) and medical devices such as ceramic pacemaker feedthroughs and high-precision implants.
How can Exentis’ technology support mass production of pharmaceutical applications?
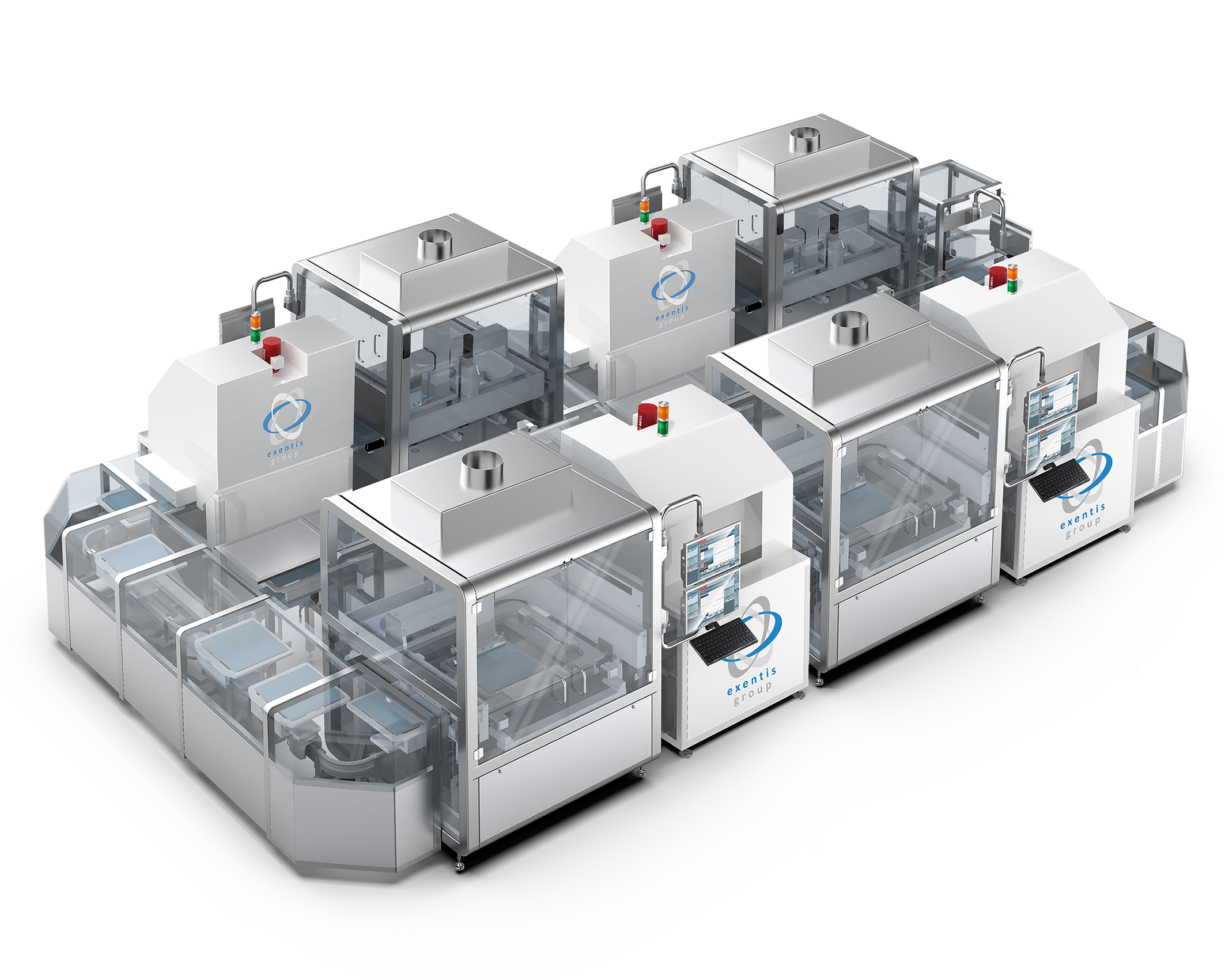
To enable mass production, Exentis’ offers a portfolio of advanced systems, including the Exentis EX431 GMP system, Exentis EX432 iflex GMP system and the Exentis EX434 iflex GMP system. These solutions are based on a cold-printing process that ensures biopharmaceuticals and sensitive drug compounds maintain their structural and functional integrity.
Exentis’ 3D screen printing technology allows pharmaceutical manufacturers to freely select and combine different active pharmaceutical ingredients (APIs) when producing tablets. The technology can incorporate up to three different APIs in a single tablet.

“This flexibility allows for the production of poly-pills and customized dosing, which are often difficult to achieve with traditional 3D printing,” Raju Willener, responsible for the Pharma/Medical unit, explains.
Additionally, as announced in the introduction of this article, Exentis’ technology can also process technical ceramics (used for medical implants such as pacemaker feedthroughs), and biodegradable polymers – which allow for controlled drug release, and ensure precise dosage delivery over time.
On a technical level, the GMP-compliant process eliminates thermal degradation, which is a major concern in pharmaceutical AM. Thanks to automation and precise drug release control, the multi-layer production system allows precise control over drug release profiles in the human body. This feature facilitates the production of immediate, extended, and sequential release tablets, optimizing therapeutic effectiveness; it also enhances galenics (the science of drug formulation) through better drug bioavailability and patient compliance.
“Unlike other 3D printing methods that are limited to prototyping or small-batch production, Exentis’ Industrialized Pharma Additive Manufacturing is a fully scalable, GMP-compliant cold-printing technology that enables mass production while preserving drug integrity, optimizing resource efficiency, and offering unparalleled formulation flexibility. Additionally, Exentis’ extensive patent portfolio reinforces its leadership in pharmaceutical additive manufacturing innovation,” Willener notes.
Complex applications in pharmaceutical and medical manufacturing

Multi-ingredient tablets, drug release formulations and high-precision medical components are considered complex applications to achieve with 3D screen printing.
Multi-ingredient tablets contain several APIs in a single production step, reducing the number of pills patients need to take daily. Custom drug release formulations allow immediate, delayed, or extended drug release profiles to be programmed within a single tablet. High-precision medical components include ceramic and polymer-based medical implants that require ultra-fine surface structures and exceptional dimensional accuracy.
A key reason behind this complexity lies in regulatory demands. Whether developing multi-ingredient tablets or high-precision implants, the pharmaceutical industry operates under strict quality control requirements. This makes batch-to-batch consistency, reproducibility, and compliance with global standards essential. As a result, these products must undergo extensive validation, biocompatibility testing, and long-term performance assessments.
Interestingly, these regulatory and compliance aspects play to Exentis’ strength: “Exentis’ GMP-certified manufacturing system ensures compliance with Pharmacopeia standards, making it viable for global pharmaceutical markets. The ability to print QR codes directly onto tablets further enhances patient compliance, enabling digital tracking and real-time information access. Screen printing structures combine the advantages of metallic properties such as good thermal conductivity, high strength and high oxidation resistance with the advantages of functional shaping, fine structures and high production quantities. Exentis’ Industrialized Pharma Additive Manufacturing platform offers robust protection against falsified medicines through its innovative tracking and authentication features, ensuring pharmaceutical security and patient safety,” the expert explains.
Other reasons that may explain this complexity include:
- Material compatibility: for multi-ingredient tablets for instance, different active pharmaceutical ingredients (APIs) may have varying physical and chemical properties (e.g., solubility, stability, melting points)
- Controlled release mechanisms in drug release formulations – a challenge Exentis addresses through tamper-proof and controlled API distribution. Indeed, Exentis’ multi-layer printing technology ensures that each tablet’s composition is unique and difficult to replicate by counterfeiters
- As well as micro-scale accuracy in high-precision components
“Exentis’ technology provides a multi-layered defense against falsified medicines, leveraging on-tablet QR codes, precise API structuring, and digital tracking to enhance drug security, protect patients, and ensure full compliance with pharmaceutical regulations,” Willener points out.
Concluding notes
 While 3D screen printing has already demonstrated strong potential in bioprinting and medical devices, its applications are gradually expanding into other industries, such as electronics and semiconductors. Exentis’ insights suggest that this technology could significantly reshape drug production and medical device manufacturing, offering new levels of precision and scalability. However, with limited concrete information on commercial implementations, we look forward to seeing real-world examples that truly showcase its capabilities.
While 3D screen printing has already demonstrated strong potential in bioprinting and medical devices, its applications are gradually expanding into other industries, such as electronics and semiconductors. Exentis’ insights suggest that this technology could significantly reshape drug production and medical device manufacturing, offering new levels of precision and scalability. However, with limited concrete information on commercial implementations, we look forward to seeing real-world examples that truly showcase its capabilities.
This article was first published in the March/April edition of 3D ADEPT Mag.
Images: Exentis Group AG – Looking for a job in the AM industry or hiring new talent? You can post job opportunities on 3D ADEPT Media for free or explore openings via our job board. Stay connected by following us on Facebook, Twitter, LinkedIn & Instagram, and subscribe to our weekly newsletter for the latest updates. Have a story to share or want to be featured in our next digital magazine issue? Send it to editor@3dadept.com !