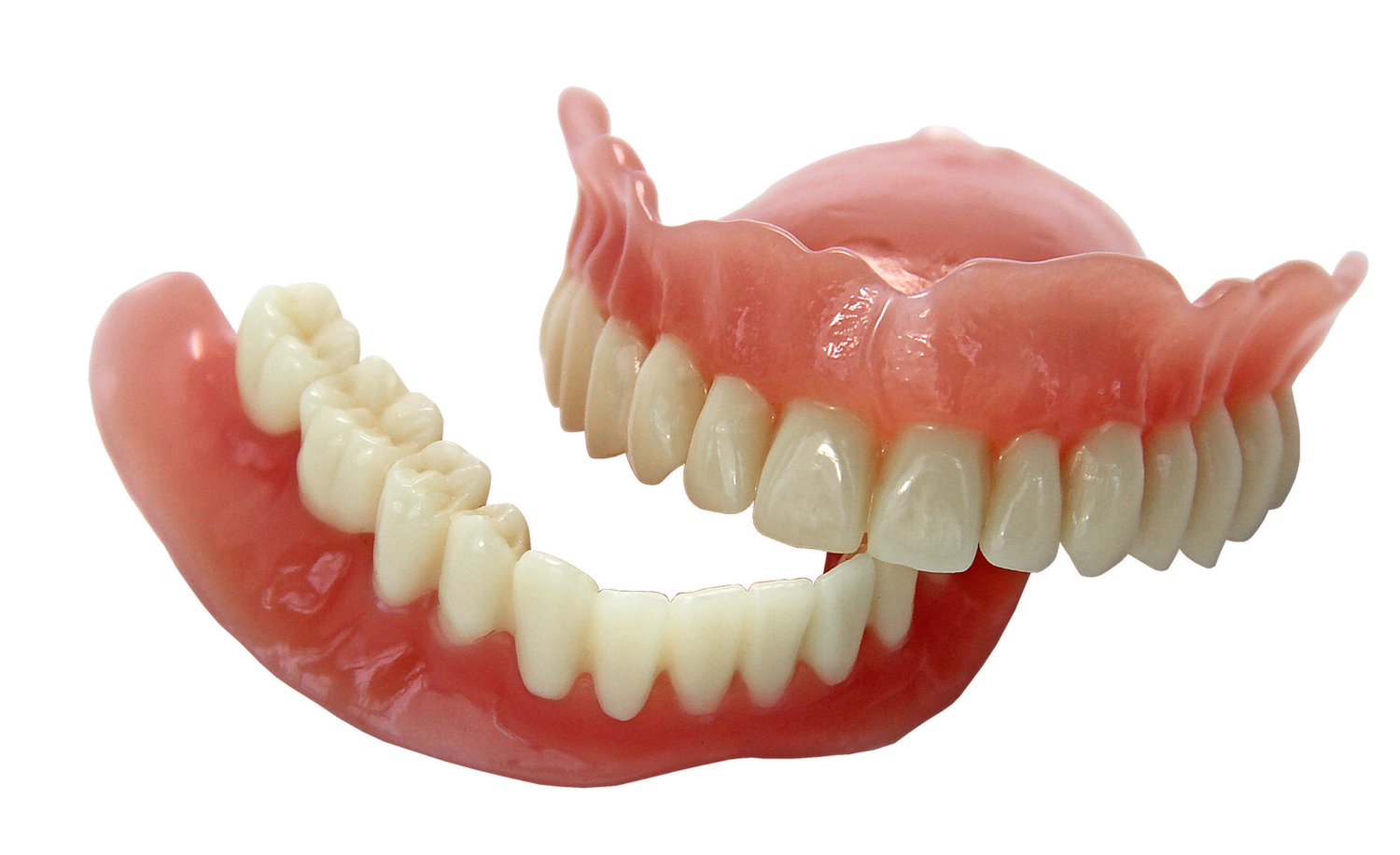
EnvisionTEC, a leading global manufacturer of desktop and full-production 3D printers and materials, today received FDA approval for its new E-Denture material for the 3D printing of lifelike dentures.
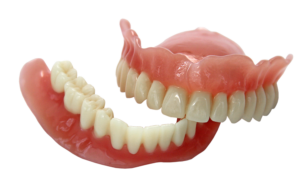
Combined with EnvisionTEC’s FDA-approved E-Dent 100 and 400 materials for the direct printing of restorations that simulate teeth, EnvisionTEC is now the only 3D printing company worldwide that offers a complete digital denture workflow solution. E-Dent is currently offered in four natural shades.
Dental labs and dentists can now combine a pink 3D printed denture base and tooth restoration into a denture that patients can wear for long-term use.

“This new material expands EnvisionTEC’s already industry-leading dental materials library,” said CEO Al Siblani. “Even more importantly, E-Denture gives our 3D printer owners the ability to print even more materials.”
Both E-Denture and E-Dent can be 3D printed on EnvisionTEC’s Vida desktop and Perfactory production machines. Owners of those machines can already 3D print a variety of dental parts with EnvisionTEC materials:
- Dental or orthodontic models (E-Model and E-Appliance)
- Castable crowns, bridges and partial denture frameworks (Press-E-Cast and E-Partial)
- Bite splints or night guards (E-Guard)
- Surgical guides (Clear Guide and E-Guide Tint)
- Indirect bonding trays for orthodontic bracket placement (E-IDB)
- Flexible gingiva masks (E-Gum)
E-Denture will be available for limited distribution in the third quarter.
//pagead2.googlesyndication.com/pagead/js/adsbygoogle.js
(adsbygoogle = window.adsbygoogle || []).push({});
(function(i,s,o,g,r,a,m){i[‘GoogleAnalyticsObject’]=r;i[r]=i[r]||function(){
(i[r].q=i[r].q||[]).push(arguments)},i[r].l=1*new Date();a=s.createElement(o),
m=s.getElementsByTagName(o)[0];a.async=1;a.src=g;m.parentNode.insertBefore(a,m)
})(window,document,’script’,’https://www.google-analytics.com/analytics.js’,’ga’);
ga(‘create’, ‘UA-94096813-1’, ‘auto’);
ga(‘send’, ‘pageview’);