Additive Manufacturing unlocks PEEK’s full potential for spinal cages, but overcoming the limitations of traditionally machined implants is key. This article explores how 3D printing enables OEMs to develop patient-specific designs, cut material waste, reduce production costs, and drive innovation in hybrid and advanced implant solutions.
PEEK (polyetheretherketon) implants in spinal surgery are making a comeback. For decades, spinal surgery has relied on machined PEEK interbody fusion devices: effective but increasingly limited with the emergence of the need for advanced design features. Additive Manufacturing solutions provider 3D Systems has shattered those limitations with an integrated manufacturing process that enables the production of a new generation of spinal fusion devices: porous PEEK spine cages out of HA- and BCP-enhanced PEEK. These implants are not just a step forward – they’re a leap, combining the potential for enhanced osseointegration, tailored mechanical properties, and unmatched design freedom. This sets a new standard for PEEK spinal implants, addressing the challenges that have held back OEMs for years.
Discover below how OEMs can leverage the technological advances of 3D Systems capabilities in manufacturing the new generation of spinal cages and why it’s poised to become the new gold standard in patient care.
PEEK in spinal surgery
PEEK has long been viewed as appealing due to its biocompatibility, radiolucency, and mechanical similarity to human bone. Traditional manufacturing methods – machining and molding – often face limitations in producing complex geometries, such as porous structures associated with enhanced osseointegration capabilities. 3D printing has stepped in to respond to these challenges. What this means for OEMs is the emergence of a new manufacturing process that can transform and expand their existing PEEK spinal cages portfolio.
- Customization and patient-specific designs: 3D printing allows us to fine-tune device properties, such as porosity and surface texture, to improve integration with bone and promote osseointegration (the bonding of bone to the implant).
- Reduced material waste and cost: 3D printing is an additive manufacturing process, meaning it only uses the material necessary for the part. This results in less material waste compared to traditional machining or molding methods.
- Competitive Advantage: AM allows to spearhead innovative projects, combining new designs and materials, allowing OEMs to differentiate their products in a competitive market.
3D Systems continues to be a trusted partner for the leaders in the spinal implants industry, thanks to decades of experience and its integrated approach to contract manufacturing: from concept and design, all the way to approval.
Why OEMs choose 3D Systems: Proprietary process for spinal cage production
What sets 3D Systems apart is its proprietary process for spinal cage production for medical device manufacturers, which redefines how PEEK spinal cages are developed and produced.
Manufacturing without compromising quality. Due to the stringent conditions required for printing PEEK, it took time for 3D printing to mature to the point where it could reliably process such an advanced material in complex geometries. That’s why hardware matters: the progress made to overcome these challenges culminated in the EXT 220 MED platform by 3D Systems, the only fully healthcare-dedicated PEEK printer in the world. It uses extrusion-based technology to produce clean PEEK spinal cages with highly intricate designs and superb finishes.
Accelerated path of innovation to market. Digital design and additive manufacturing of spinal cages means we can rethink implant design by choosing from various lattice structures, determining mechanical properties for the cage. For example, by strategically adding dense areas, the stiffness and strength of the cage can be precisely tailored to meet specific surgical and patient needs. This innovative approach allows unparalleled control over implant properties, ensuring that each cage is optimized for load distribution and fusion outcomes. This enables the production of spinal cages that are functionally superior, future-proof, and more adaptable to evolving clinical requirements.
Leveraging such a predictable process offers an opportunity for OEMs to join forces and work with 3D Systems to develop new, innovative applications. For example, hybrid cages: combining a PEEK body with a titanium endplate that can offer enhanced structural and integration properties. Beyond that, 3D Systems also facilitates the production of trial implants for surgical testing and carbon-reinforced PEEK cervical plates, providing a comprehensive solution for all elements surrounding spinal implants.

Based on extensive experience working on minimizing the time it takes innovative solutions to impact patient lives, the 3D Systems team works closely with OEMs to help them accelerate the time of a new device to market.
The new generation of PEEK spinal cages for proven stability and faster recovery. The AM capabilities from 3D Systems have enabled a paradigm shift when it comes to PEEK spinal cages.
- From dense PEEK cages with a graft window to open porous structure cages to promote bone integration
- From using mostly virgin, inert PEEK to using osseointegrative PEEK variants (Biphasic Calcium Phosphate/Hydroxyapatite enhanced) that are claimed to promote bone growth
- From occasionally applying titanium (Ti) coatings on spinal cages for enhanced osseointegration to designing more tailored structural solutions that have dense areas taking the load only where needed
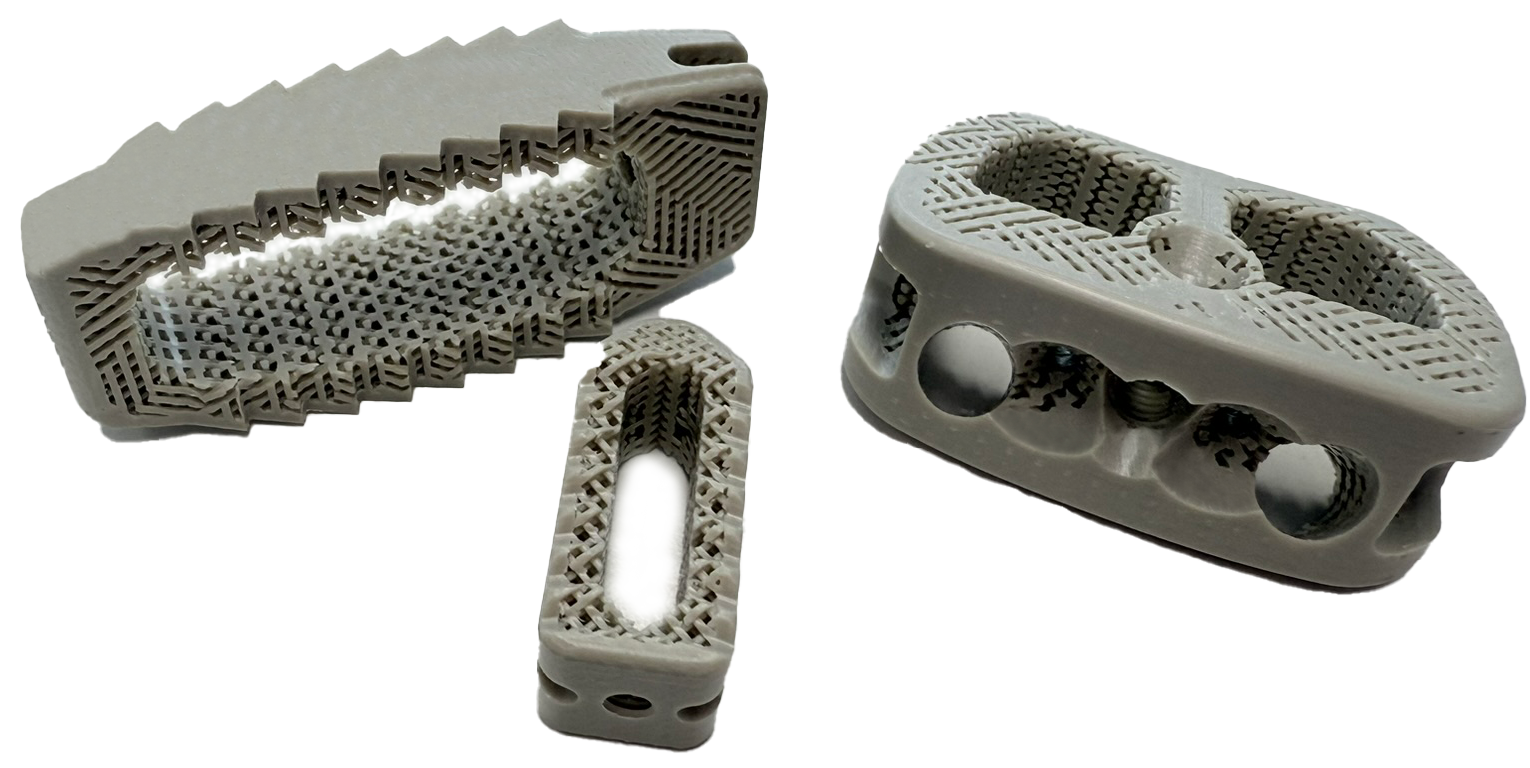
The new generation of PEEK spinal cages not only offers improved structural design, but they are also claimed to promote enhanced clinical outcomes.
Altogether, 3D Systems has leveraged decades of experience in the medical device industry to set up a stable, reproducible process which enables the production of high-quality spinal cages – every time, all the time. This manufacturing process allows us to provide implants with tailored porosity for enhanced fusion rates while ensuring the structural durability needed for successful outcomes. The sheer ability for such spinal cages to be manufactured sets a new standard for implants and patient care.