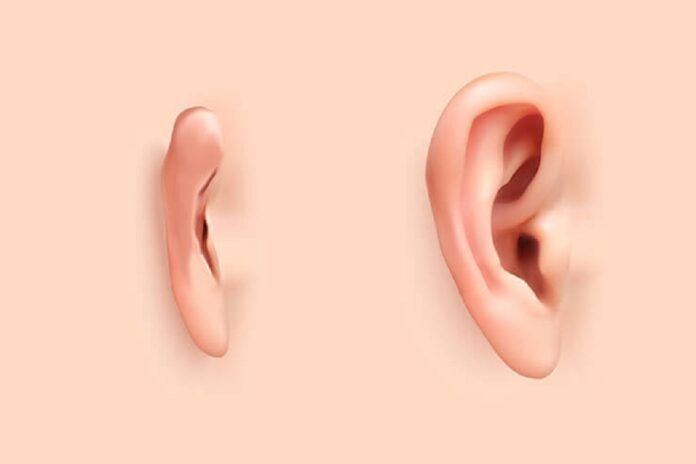
3DBio Therapeutics (3DBio), a clinical-stage regenerative medicine company, and the Microtia-Congenital Ear Deformity Institute announced they have conducted a human ear reconstruction using the AuriNovo™ implant, an investigational, patient-matched, 3D-bioprinted living tissue ear implant. The groundbreaking reconstructive procedure in the first-in-human Phase 1/2a clinical trial is evaluating the safety and preliminary efficacy of AuriNovo™ for patients with microtia, a rare congenital deformity where one or both outer ears are absent or underdeveloped. Microtia affects approximately 1,500 babies born in the US annually. This transformational implant procedure was performed by a team led by Arturo Bonilla, M.D., a leading pediatric ear reconstructive surgeon specializing in microtia and the founder and director of the Microtia-Congenital Ear Deformity Institute in San Antonio, Texas.
AuriNovo™ is a patient-specific, living tissue implant created using 3D-bioprinting technology for surgical reconstruction of the outer ear in people born with microtia Grades II-IV. AuriNovo™ is designed to provide a treatment alternative to rib cartilage grafts and synthetic materials traditionally used to reconstruct the outer ear of microtia patients. The U.S. Food and Drug Administration (FDA) has granted AuriNovo™ Orphan Drug and Rare Pediatric Disease Designations.
“As a physician who has treated thousands of children with microtia from across the country and around the world, I am inspired by what this technology may mean for microtia patients and their families,” said Dr. Bonilla. “This study will allow us to investigate the safety and aesthetic properties of this new procedure for ear reconstruction using the patient’s own cartilage cells. My hope is that AuriNovo™ will one day become the standard-of-care replacing the current surgical methods for ear reconstruction requiring the harvesting of rib cartilage or the use of porous polyethylene (PPE) implants. The AuriNovo™ implant requires a less invasive surgical procedure than the use of rib cartilage for reconstruction. We also expect it to result in a more flexible ear than reconstruction with a PPE implant. The AuriNovo™ living tissue implant is designed to provide a better solution for patients born with microtia by transforming their appearance and building their confidence and self-esteem.”
Daniel Cohen, Ph.D., 3DBio Chief Executive Officer and Co-founder, and his team have built a comprehensive, proprietary technology platform to deliver living tissue implants to patients. “This is a truly historic moment for patients with microtia, and more broadly, for the regenerative medicine field as we are beginning to demonstrate the real-world application of next-generation tissue engineering technology. It is the culmination of more than seven years of our company's focused efforts to develop a uniquely differentiated technology platform meeting the FDA’s requirements for therapeutic manufacturing of reconstructive implants,” said Dr. Cohen. “We believe that the microtia clinical trial can provide us not only with robust evidence about the value of this innovative product and the positive impact it can have for microtia patients, but also demonstrate the potential for the technology to provide living tissue implants in other therapeutic areas in the future.”
“Our initial indications focus on cartilage in the reconstructive and orthopedic fields including treating complex nasal defects and spinal degeneration,” continued Dr. Cohen. “We look forward to leveraging our platform to solve other high impact, unmet medical needs like lumpectomy reconstruction and eventually expand to organs.”
In addition to the Microtia-Congenital Ear Deformity Institute, the AuriNovo™ Phase 1/2a clinical trial is enrolling patients at Cedars-Sinai Medical Center in Los Angeles, CA under the direction of John Reinisch, M.D., Director of Craniofacial and Pediatric Plastic Surgery.
Remember, you can post job opportunities in the AM Industry on 3D ADEPT Media free of charge or look for a job via our job board. Make sure to follow us on our social networks and subscribe to our weekly newsletter : Facebook, Twitter, LinkedIn & Instagram ! If you want to be featured in the next issue of our digital magazine or if you hear a story that needs to be heard, make sure you send it to contact@3dadept.com