Despite the capabilities of Additive Manufacturing, the road to full-scale production has often been a hurdle that slows its broader adoption, especially in the healthcare sector. Medical device manufacturers have largely explored AM through customized and on-demand parts, rather than serial production. But Lincotek Medical is changing that narrative. With over 100,000 medical parts 3D printed annually, the company is no longer experimenting with AM; it’s scaling it. In the article below, we caught up with Mukesh Kumar, Technology and R&D Director at Lincotek Medical, to better understand their journey toward industrializing AM for medical applications.
While for many, the additive manufacturing journey of Lincotek dates back to 2019, when Unitedcoatings Group, a global contract manufacturer for Industrial Gas Turbine, Aviation and Medical Device applications, launched Lincotek Additive, the reality is, the company started its journey in the medical sector in 2006 with Lincotek Medical, focussing exclusively on the orthopedic sector.
With AM used across several divisions of the company, Lincotek Additive is no longer a single division of the company.

“At Lincotek, Additive Manufacturing expertise is not limited to medical applications. An entire team dedicated to non-medical solutions operates under Lincotek’s Surface Solutions Division, primarily serving the Energy and Aerospace sectors from our facility in Spreitenbach, Switzerland. While the Medical and Industrial AM teams focus on their respective markets, they work closely together, sharing insights, best practices, and technical advancements across divisions.
This unique environment fosters cross-fertilization of ideas and expertise, enabling Lincotek to stay at the technological forefront in each of our niche markets. By leveraging this internal synergy, we’re able to deliver best-in-class solutions to our customers,” Kumar explains from the outset.
AM as a means to an end
With facilities in Memphis, TN, USA, and Trento, Italy, Lincotek operates about 40 3D printers from EOS, 3D Systems, Concept Laser (now Colibrium Additive), and Renishaw. This production environment indicates a focus on manufacturing medical parts using LPBF and Electron-beam additive manufacturing. The company is also investigating the application of binder jetting and other production technologies.
“By combining conventional and additive manufacturing under one roof, we offer end-to-end solutions — from design and 3D printing to the finished part. We see AM not as a standalone technology, but as one step in a broader, integrated process. What makes us unique is our ability to seamlessly connect every stage, with complementary processes already embedded in the design and development phases,” the Technology and R&D Director explains.
This production environment refocuses the debate on the increasing use of metal 3D printing, especially LPBF, for medical applications.

From our coverage, we realized that due to its ability to meet the functional and mechanical requirements of the parts produced, LPBF is often a good candidate for orthopedic implants – a key application in Lincotek Medical’s portfolio. Indeed, materials like titanium alloys (e.g., Ti-6Al-4V) often shine through their ability to meet strict standards for biocompatibility, strength, and durability.
Kumar emphasizes these reasons and more:
“We already had deep expertise in managing implants and understood the quality and regulatory requirements placed on total solution providers and OEMs. We were also in direct contact with decision-makers at OEMs. So, transitioning to Additive Manufacturing with Ti6Al4V — and to some extent CoCrMo — was a natural fit.
Our focus on metal 3D printing, specifically Laser Powder Bed Fusion (LPBF) for medical applications, has been driven by several key factors, particularly when compared to the more common use of polymer 3D printing in the same field.
Product mix run: Metals like titanium, stainless steel, and cobalt-chrome alloys offer superior mechanical properties such as strength, durability, and wear resistance. These attributes are essential for implants and surgical instruments that must perform reliably over time. While polymers offer flexibility and easier manufacturability, metals provide the necessary mechanical strength and longevity that are vital in medical settings. As a leader in contract manufacturing, we primarily print implants in titanium and titanium alloys, with strong expertise in LPBF using CoCr and stainless steel as well.
Industry demand and regulatory acceptance: Although polymer 3D printing remains widespread for prototyping, the medical device industry is increasingly turning to metal 3D printing for end-use parts that meet strict regulatory standards. Metal implants have already received FDA approvals, and the regulatory pathways for metal AM in healthcare are becoming clearer, making it an attractive avenue for investment and development.”
That said, beyond prototyping, polymer 3D printing is also ideal for applications such as surgical guides and non-implantable devices where strength and biocompatibility are less critical.
At Lincotek, plastic (polymers) and wax printers often support the need for OEMs in clinical use, where non-implantable parts are required.
Key applications
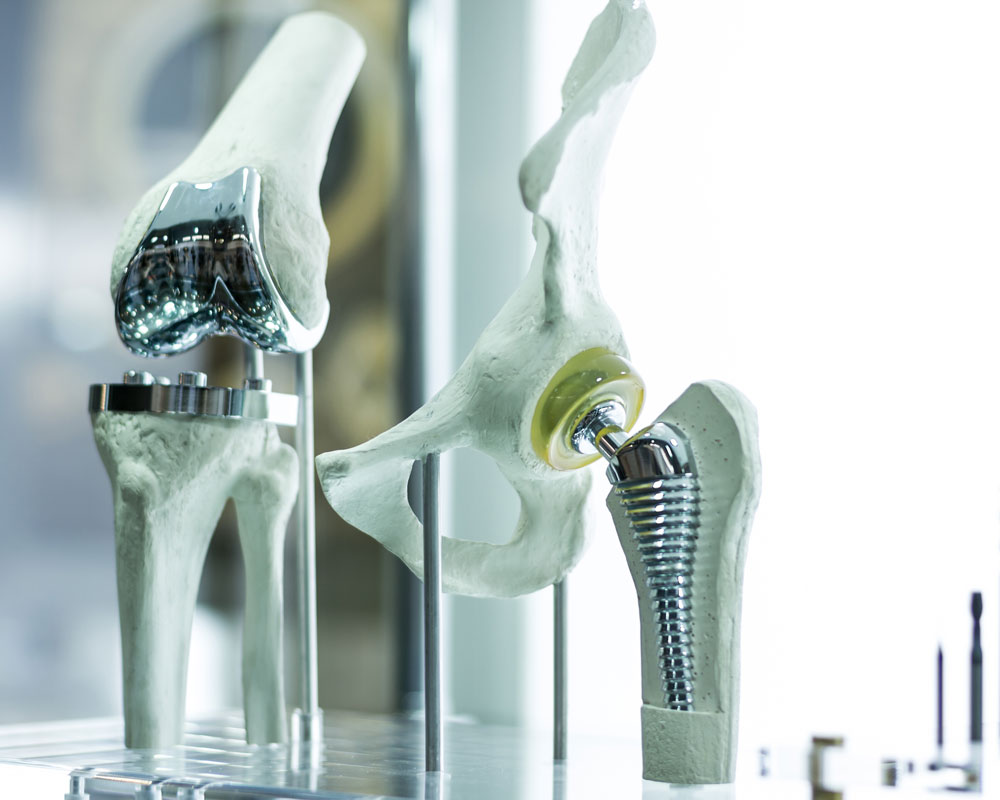
As outlined above, Lincotek Medical has built extensive expertise in the manufacture of orthopaedic devices. The company claims it is making “major inroads with spinal and F&A space”. From joint and bone implants to spinal implants and custom prosthetics, Lincotek Medical aims to deliver a comprehensive range of solutions to medical device OEMs.
Orthopaedic medical devices with porous structures facilitate bone ingrowth. According to Kumar, when implanted correctly, these solutions mitigate clinical risks and significantly decrease the time from purchase order to the availability of parts on the hospital shelf.
All these implants can also be tailored to fit the specific anatomy of a patient, improving both the fit and functionality, and ensuring long-term success rates.
“More broadly, we’re now in full production with additively manufactured tibial trays, patellas, and cones. We’ve also conducted extensive R&D to validate the feasibility of producing titanium femoral components with porous structures using AM. These components can be further enhanced with coatings such as TiN, TiNbN, or other hard coatings, improving articulation and wear performance. We believe this approach will help OEMs address concerns associated with CoCr-based components while supporting cementless fixation — ultimately improving patient care.
This progress builds on our extensive experience with titanium alloy acetabular shells featuring porous, interconnected outer layers. These designs provide primary fixation due to their high grip and also promote long-term secondary fixation, thanks to optimized porosity and pore sizes. What makes this design particularly successful is its adaptability across a wide range of components — from tibial plates and shoulder implants to spinal devices — combined with industrial flexibility, a broad size range, and proven clinical performance,” Kumar enthuses.
Challenges and outlooks
 Regulatory compliance is a common challenge faced by almost all medical device manufacturers, and Lincotek is no exception. In its compliance strategy, the company ensures that the materials it uses are certified according to international standards such as ASTM F136 (for titanium alloys) and ISO 10993 (for biocompatibility).
Regulatory compliance is a common challenge faced by almost all medical device manufacturers, and Lincotek is no exception. In its compliance strategy, the company ensures that the materials it uses are certified according to international standards such as ASTM F136 (for titanium alloys) and ISO 10993 (for biocompatibility).
To ensure device certification and risk management, the company conducts extensive preclinical and clinical testing as well as compliance with regulatory pathways like the FDA’s 510(k) or CE marking process in Europe.
“Lincotek Medical adheres to the strictest regulatory frameworks, such as the FDA’s Quality System Regulation (QSR) for medical devices (21 CFR Part 820) and the European Medical Device Regulation (MDR). They maintain a robust risk management process (per ISO 14971) to ensure that potential hazards are identified, assessed, and mitigated throughout the product lifecycle.
As for quality control and repeatability, Lincotek’s Medical Division implements a multifaceted strategy that spans rigorous process control, advanced technology, standardized workflows, comprehensive testing, and robust documentation. By adhering to international quality standards like ISO 13485 and using advanced monitoring and automation technologies, Lincotek ensures that each 3D-printed medical device is produced with the highest level of precision, consistency, and regulatory compliance,” Kumar concludes.
Kumar’s responses have been edited for brevity and clarity.
*We curate insights that matter to help you grow in your AM journey. Receive them once a week, straight to your inbox. Subscribe to our weekly newsletter. Read the latest healthcare edition of 3D ADEPT Mag here.





