While powder reuse is a legitimate strategy embraced by various industries aiming to optimize sustainability and reduce costs, the healthcare sector must go far beyond these considerations. For medical 3D printed devices, patient safety and clinical performance are paramount. To what extent can powder reuse be compatible with the stringent requirements of medical applications? And are the criteria for this approach fundamentally different from those in other verticals?
(Almost) all metal 3D printing technologies (Directed Energy Deposition (DED), Binder Jetting, Selective Laser Melting (SLM) or Laser Powder Bed Fusion (L-PBF), Selective Laser Sintering (SLS), and Electron Beam Melting (EBM)) require effective powder management strategies, including recycling. Each of these processes presents unique challenges. For instance, users of direct melting technologies like L-PBF, EBM, and DED must contend with residual stresses in the material, while those working with Binder Jetting face issues related to residual binder in recycled powders. These factors can compromise the quality of new builds and often call for additional cleaning or reprocessing after manufacturing.
Given the growing adoption of L-PBF in the healthcare and medical sectors, this discussion will focus primarily on the implications of powder reuse in that context. Key elements that will be explored include:
- Changes in powder characteristics during powder reuse
- Powder mixing strategies
- Data assessed in powder reuse
First and foremost, “the term “recycled powder” is used loosely in the AM industry. Normally, recycled powder means powder that is “recycled” during the printing process by using integrated or standalone powder recycling centres (PRC) that involve mainly sieving. PRCs take the unused powder from a print cycle in the 3D printer and sieve it to the desired particle size to remove any agglomerated particles. This system guarantees that only the particle size goes back to the printer,” Sunil Badwe, VP of R&D at Continuum Powders clarifies. This article will focus on powders recycled during the printing process.
In general, aluminum alloys (e.g., AlSi10Mg), nickel-based superalloys (e.g., Inconel 718, 625), stainless steels (e.g., 316L), Cobalt-Chromium (CoCr) alloys, and Titanium alloys (especially Ti-6Al-4V) are metallic powders that are often subject to a powder reuse approach. In this list, stainless steels (e.g., 316L), Cobalt-Chromium (CoCr) alloys, and titanium alloys are often considered as the ideal material candidates for healthcare 3D printing applications.

Indeed, titanium alloys have proven to be ideal for orthopedic implants, dental prostheses, whereas Cobalt-Chromium alloys often serve the needs of dental frameworks, joint replacements, and cardiovascular implants. Stainless steels, on the other hand, are often explored for surgical tools and implants.
“The optimal reuse strategy depends on both the powder material and the application. To define an effective strategy, it’s crucial to understand how key powder properties evolve through reuse and how those changes impact the final part quality. When done right, an effective powder reuse can unlock significant cost savings and sustainability benefits,” Tim Wischeropp, CEO, amsight GmbH, states from the outset.
Agreeing with him, Dr.-Ing. Gregor Graf, Head of Technology at Rosswag Engineering, adds that for each material, customized reuse protocols are required. “Titanium alloys, for example, are highly reactive to oxygen and moisture, and require sensor-based drying and storage. Stainless steels are more forgiving.”
That said, it’s crucial to ensure that a high-quality powder is utilized from the very first use. This will influence flowability, which will affect the quality of the AM process and the mechanical properties of the 3D printed part. In general, factors that determine the quality of a metallic powder include: particle size and shape, density, roughness, chemical composition, and the presence of impurities in the material.
A powder reuse approach goes beyond these characteristics.
Changes in powder characteristics during powder reuse

A key emphasis is placed on the characteristics of the powder due to its specific behavior within the metal 3D printing process.
Since only a small portion of the powder in each build is fused into a part, the rest is left unmelted and often reused. If this leftover powder were automatically classified as contaminated and unsuitable for reuse, the cost of producing 3D printed parts would rise significantly, making the technology economically unviable for many applications.
In theory, “process parameters should be adjusted when using recycled powder, as its properties can change over time – for instance, in flowability, oxidation level, or particle distribution. However, in practice, such parameter optimization is resource-intensive and is typically carried out only for critical applications, such as medical or aerospace parts.

At Rosswag Engineering, we address this challenge through a tool-assisted strategy. Our MES AddiPlan tracks powder reuse cycles, environmental data (e.g., via retrofitted humidity sensors in LPBF machines), and batch-specific quality metrics. This allows us to identify when parameter deviations would have a significant impact on part quality.
For critical use cases, we draw on our in-house parameter development expertise and leverage AddiMap, the world’s first marketplace for LPBF process parameters. This lets us efficiently select or adapt parameter sets based on material, machine type, and target properties – saving time while ensuring reproducibility.
In most cases, our pre-qualified standard parameters remain valid due to tight process control. But when needed, our digital infrastructure ensures we can rapidly and precisely intervene,” Rosswag’s Head of Technology points out.
Rosswag’s approach emphasizes the disparity that may exist between the numerous metal 3D printers available in the market and their powder handling systems. For instance, Renishaw’s AM250 system allows for manual addition of used powder directly into the top-mounted silo, whereas other systems employ more automated and closed-loop processes. The EOS M 290, for example, utilizes the IPCM M pro system, which facilitates semi-automated, dust-free powder handling. Excess powder is vacuum-conveyed from the build chamber to the IPCM M pro, where it is sieved under an inert gas atmosphere.
These examples already shed light on the type of powder mixing strategies that can be used in powder reuse.
Powder mixing strategies

Essential when reusing powder across multiple builds, mixing strategies help to maintain consistent material properties, flowability, and chemical composition—all essential for producing high-quality, certifiable medical 3D printed parts.
While each technique brings its share of strengths and weaknesses that should constitute the focus of another article, one can already identify two main techniques used in the industry:
The first one involves blending virgin material with leftover excess. Since it often reduces the oxygen levels in most metal alloys, it is not always ideal for creating recycled powder of high quality, ideal for critical applications such as medical devices.
The second strategy involves using a single batch of powder repeatedly, build after build, until it can no longer produce parts that meet the required specifications. In this approach, the unused powder is sieved after each build to remove contaminants and large particles, then returned to the machine for reuse. This cycle continues until the remaining powder is either insufficient to complete a build or has degraded to the point of being unsuitable.

“Traditional powder reuse by sieving mainly prevents oversized powder particles from returning to the systems; however, it does not have the ability to prevent any foreign object debris (FOD), burned powder, oxidized powder, or chemically degraded powder. Continuous monitoring of the material properties of printed materials is necessary to track property degradation. This control is important in determining or limiting the reuse cycles of the material. Unfortunately, as the powder can vary from lot to lot, the number of reuse cycles also varies accordingly,” Sunil Badwe explains. According to Continuum Powders, the PSD of recycled (sieved) powder is little changed after the first pass. In other words, the fine particle content (normally <10m) ) removed after the first “reuse”.
As a part manufacturer, Rosswag uses batch blending strategies and target particle distribution windows optimized for its SLM®280 twin machines. With AddiPlan’s powder module, they track and mix powder batches according to their reuse history, humidity, oxygen content, and granulometry. These datasets are vital for defining machine-specific blends for consistent performance.
That’s the reason why most experts including Graf agree with the fact that powder reuse affects:
- Particle shape and distribution (more satellites, fines, or agglomerates),
- Oxidation and moisture absorption, leading to reduced flowability and print stability,
- Chemical degradation (e.g., evaporation of low-melting elements),
- Mechanical behavior in final parts.
“To mitigate this, we leverage sensor-backed drying and sieving systems, as described in our patent [DE102017009833B3], to ensure powder condition matches application demands. Scientific insights (e.g., Lanzutti & Marin, 2024) further validate these risks and mitigation strategies,” he says.
These characteristics somehow influence the type of data that must be assessed – and the type of powder mixing strategy that should be leveraged.
Data assessed in powder reuse
The quality of a 3D printed medical product is significantly influenced by the characteristics of the raw materials employed. The data required for analysis in this context varies based on the software solutions utilized.
In addition to the material used, Tim Wischeropp, CEO, amsight GmbH, emphasizes the type of application to achieve. Indeed, a simple part may lead to more opportunity for material reuse than a complex part, which would involve considering more aspects of the manufacturing process in general.

“However, across all metal powders, consistently monitoring the chemical composition—especially oxygen content and humidity levels, is critical. These factors directly influence the integrity and performance of the final component,” Wischeropp underscores.
Speaking of how their software solution helps to monitor and manage powder reuse to ensure compliance with medical device regulations, he adds:
“Regulatory bodies like the FDA require continuous monitoring of powder materials, including properties such as chemical composition, to ensure they remain within defined limits. Manufacturers must also demonstrate a clear understanding of how powder aging during reuse affects material performance—and prove that it does not significantly impact the final part quality. Our powder tracking tool simplifies this process by enabling users to monitor the entire lifecycle of their powder materials. It tracks and visualizes powder cycles, batch mixing, and property changes over time. Most importantly, it links powder data to part quality outcomes, empowering users to make informed, data-driven decisions. This helps optimize powder reuse strategies while maintaining both regulatory compliance and high product quality.”
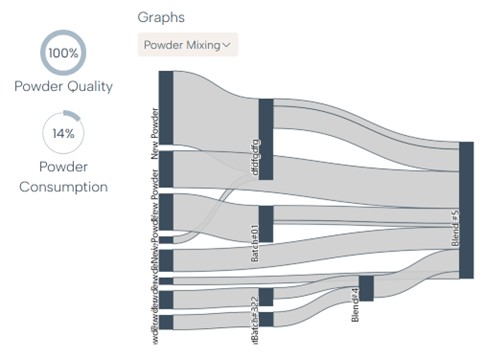
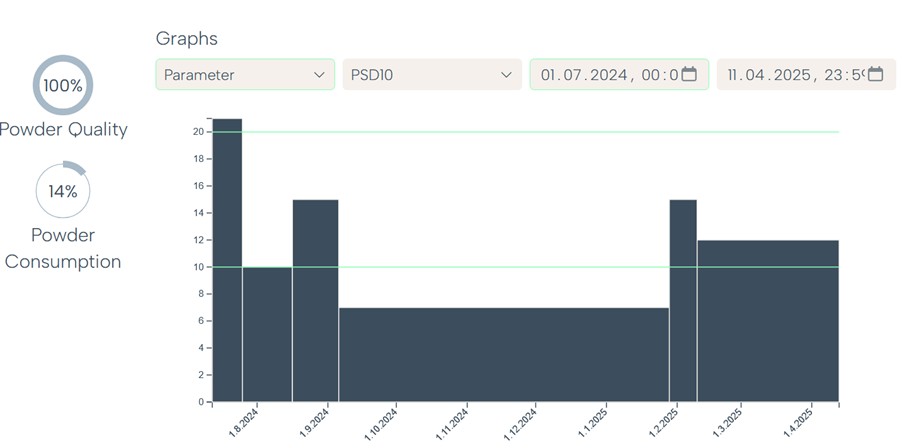
In addition to chemical composition and contamination levels, Rosswag’s expert recommends analyzing data related to particle morphology and distribution, moisture content, oxygen and nitrogen values as well as powder flow and density metrics.
The Manufacturing Execution System (MES) software platform stores and analyzes data for complete traceability. It serves as a central digital twin for every production step, tracking powder reuse cycles, environmental conditions (humidity, gas purity), machine parameters, and QA data from tensile tests and CT scans. This data structure aligns perfectly with ISO 13485 and MDR documentation requirements.
“Data is therefore fundamental. Without a robust system for managing and analysing powder-related data, it’s nearly impossible to establish effective quality gates or optimize reuse strategies. The return on investment for proper powder management can be seen in just a few weeks, thanks to the significant cost savings it enables,” amsight’s CEO concludes.
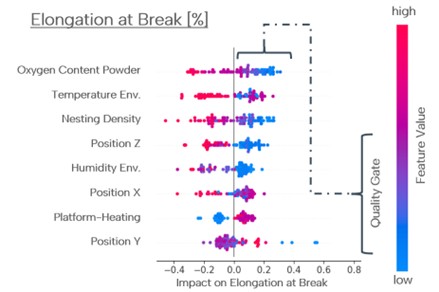
Concluding notes
When achieved properly, powder reuse ultimately reduces the cost of the final part, but in healthcare applications of vital importance, cost is not a priority factor. Particle morphology, flowability, and chemical composition are. Furthermore, with the possible increase in oxygen content during reuse cycles, particularly for titanium alloys, this method requires parts to undergo stricter quality controls.
That said, make sure you stick to tight internal reuse protocols or only use virgin powder to ensure traceability and consistency in case of uncertainty regarding the standard processes for characterizing and certifying reused powders.

Editor’s notes:
This dossier has first been shared in the 2025 healthcare edition of 3D ADEPT Mag. Discover the entire issue here. Featured image: Rosswag Engineering.
Three companies have been invited to share their expertise on this topic:
amsight focuses on helping manufacturers turn complex data into actionable insights—improving part quality, reducing costs, and meeting the stringent demands of industries like medical. The company develops a software solution that helps to optimize the Additive Manufacturing processes, from feedstock to final part.
Rosswag Engineering combines deep metallurgical expertise, smart software and platform innovation. A key initiative is AddiMap, a digital process parameter marketplace for Laser Powder Bed Fusion. It allows engineers to share, buy, and apply validated parameter sets – dramatically reducing the time and cost of process development.
AddiMap is to be integrated with platforms like Autodesk Fusion 360 to streamline the workflow from design to production. This accelerates the industrialization of metal AM and democratizes access to high-performance process knowledge across the industry.
Combined with its MES AddiPlan, real-time sensor integration (like its retrofitted humidity monitors), and its strategic investment in the powder sourcing platform qualloy, the company is building a connected ecosystem – one where quality, traceability, and efficiency are the standard.
Continuum Powders offers a powder that is newly manufactured using recycled feedstock that can involve used powder, scrap parts, machine shop scrap etc. This is not a “recycled powder” but a virgin powder that is manufactured using recycled feedstock. This powder is equivalent to any gas or plasma atomized powder. The powder characteristics are equivalent to other gas and plasma atomized powders.






