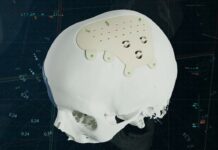
If medical implants such as pacemakers, artificial joints, and dental implants – to name a few, have significantly enhanced the quality of life for millions of people worldwide, their challenges for long-term adoption almost always remain the same and often come down to implant durability, compatibility, and infection risk.
The good news is, advancements in materials and manufacturing techniques can help address these hurdles. With Additive Manufacturing especially, the production of implants is often achieved with materials that fall into two main categories: polymer and metals. Given the fact that each of these material categories requires a specific AM process and manufacturing chain in general, how do you choose the ideal material for a medical implant?
Identifying and assessing what plays to polymer and metal 3D printed implants’ strengths is the goal the next Additive Talks session ambitions to address. Entitled “Key differences between polymer and metal 3D printed implants”, this Additive Talks virtual table will take place on Wednesday, July 17th, from 03.30 pm to 04.30 pm Central European Time (from 9.30 am to 10.30 am New York Time/ EDT) and will gather:
Kurt Jacobus, Co-founder, Chief Executive Officer and Chairman at restor3d. For the past two decades, Jacobus has dedicated his professional pursuits to improving human lives by working at the intersection of new technology and orthopedic medical devices. His efforts have led to the commercialization of multiple novel technologies resulting in the launch of more than 50 distinctive products which have improved the lives of hundreds of thousands of patients. This impact has led to multiple transactions for the businesses he has led, most recently the sale of MedShape to Enovis and ConMed and the sale of Vertera Spine to NuVasive. 2024 marks his 19th consecutive year of serving as the CEO of an orthopedic device company, and 19th consecutive year of year-over-year sales growth.
restor3d specializes in patient-specific, 3D printed, musculoskeletal implants. The company is driven by the belief that every patient deserves personalized care. The company holds proprietary expertise in 3D printing of osseointegrative materials, AI-based planning and design automation tools, as well as digital health solutions that enable to provide seamless data-backed care to optimize individual patient outcomes. Alongside its customers, restor3d is reimagining the musculoskeletal reconstruction landscape.
Marc Knebel, Head of Medical Systems Market Segment within Evonik´s High Performance Polymers Business Line. With twenty-five years of experience in the medical industry, Knebel has honed his skills in project management, customer relations, product launches, and leading diverse teams on a global level.
For more than 20 years, multinational specialty chemicals company Evonik has been manufacturing high-performance polymers for Additive Manufacturing. The specialty chemicals company offers the industry’s most extensive portfolio of biomaterials used in the fabrication of 3D printed medical device parts – designed for temporary and permanent body contact. All VESTAKEEP PEEK filaments can be processed in common extrusion-based 3D printing technologies such as fused filament fabrication (FFF). Evonik’s new VESTAKEEP® Fusion product line stands for the next generation of PEEK with osteoconductive properties. In addition, Evonik introduces the first carbon-fiber-reinforced PEEK filament for high-strength implant applications. Evonik’s portfolio also includes the RESOMER® line of bioresorbable filaments, powders and granules for implantable medical devices.
Does this look like a conversation that you do not want to miss?